Hematopathologists, molecular pathologists, and hematologic oncologists at Dana-Farber/Brigham and Women’s Cancer Center have developed and implemented a new genetic test that is improving and accelerating the diagnosis and management of patients with leukemias, myelodysplastic syndromes, and myeloproliferative disorders.
One of the most comprehensive assessment tools available for rapid assessment of hematologic malignancies, the Rapid Heme Panel employs next-generation sequencing technology to assay over 1,300 selected regions in 95 genes that are frequently mutated in myeloid and lymphoid malignancies. The Panel helps to:
- Establish and refine diagnosis, including distinguishing between underlying malignant or non-malignant disorders
- Rapidly detect genes with important prognostic implications that are useful in treatment planning, such as deciding between consolidation chemotherapy or stem cell transplantation
- Identify patients who may be eligible for clinical trials, including early-phase trials of new targeted therapies
“This powerful test not only provides more rapid results than we received from outside labs in the past, but it delivers a vast array of additional information that is highly valuable in making decisions regarding the best course of treatment for each patient,” said Jon Aster, MD, PhD, director of Hematopathology at Dana-Farber/Brigham and Women’s Cancer Center.
 Rapid Heme Panel analysis is performed at the Center for Advanced Molecular Diagnostics (CAMD) in the Department of Pathology at Brigham and Women’s Hospital, and results are available in three to seven days. Designed specifically to meet the needs of specialists who are caring for patients with hematologic malignancies, the Rapid Heme Panel was developed by a multidisciplinary team.
Rapid Heme Panel analysis is performed at the Center for Advanced Molecular Diagnostics (CAMD) in the Department of Pathology at Brigham and Women’s Hospital, and results are available in three to seven days. Designed specifically to meet the needs of specialists who are caring for patients with hematologic malignancies, the Rapid Heme Panel was developed by a multidisciplinary team.
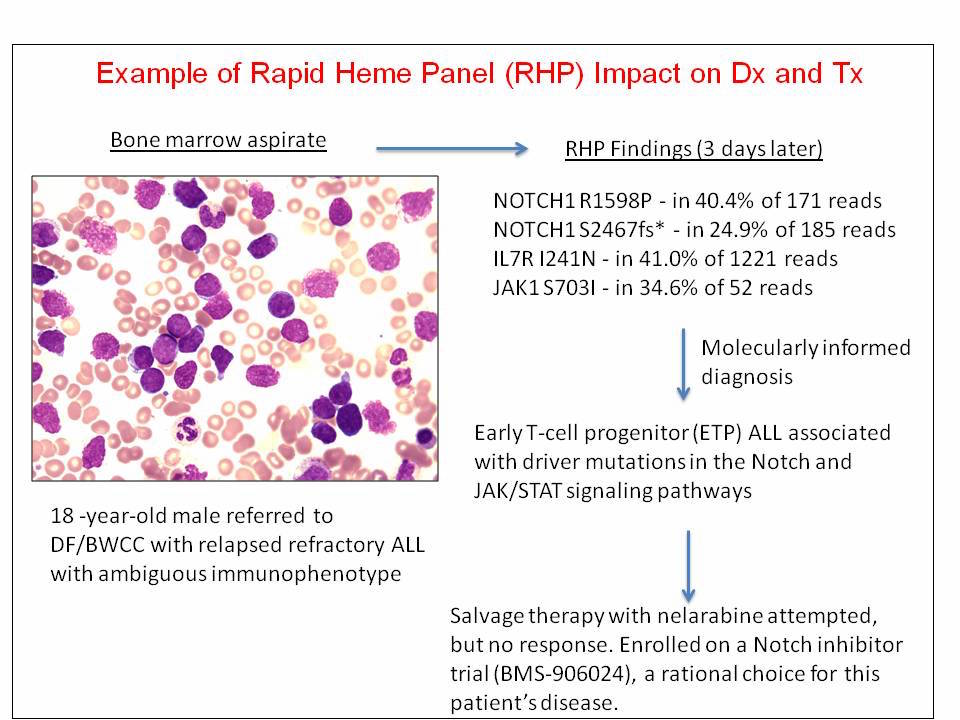
Results from the Rapid Heme Panel are being used to identify patients for enrollment in clinical trials of targeted therapies, including inhibitors of NOTCH, IDH1, and IDH2.
The Rapid Heme Panel also provides an opportunity for researchers to better understand the pathophysiology of hematologic malignancies and further identify significant prognostic indicators and therapeutic targets.
“Over time, we will be able to track the frequency of certain mutations and identify and target so-called founder mutations that are present at the root of the disease,” said Dr. Richard Stone, MD. “This will be useful in developing more effective therapies.”
Jon Aster, MD, PhD discusses the new DNA-based test which relies on relatively new technology that analyzes the 95 genes which are among the most commonly mutated genes in blood cancers such as acute leukemias, myelodysplastic syndromes and myeloproliferative neoplasms.


