Precision cancer medicine is an evolving approach to cancer care that aims to leverage new knowledge regarding the pathogenesis of cancer to more precisely target therapy. We now know most or all cancer results from abnormal genes or gene regulation. The cause of these changes can be environmental, such as smoking, spontaneous, such as a result of mutations or changes in genes, or genetic alterations that are inherited.
Clinicians at the Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) are now armed with specialized tests, creating a precise “tumor profile” of genetic changes for each patient’s cancer that makes it possible to identify the most important mutations. The results of these tests can aid us in selecting specific therapeutic agents to precisely treat each individual’s cancer.

Neal I. Lindeman, MD, director of the Center for Advanced Molecular Diagnostics at Brigham and Women’s Hospital processes tumor sample data for the Profile project

Qingsong Liu, PhD, working in Prof. Nathanael Gray’s lab is developing small molecule kinase inhibitors via chemical biology approach to target dysregulated kinases as anti-cancer therapy
This tumor profile of genomic changes helps physicians more accurately diagnose the subtype of a patient’s cancer, predict its behavior, and help select precisely targeted treatments. In essence, if we know which genes are altered in any given cancer, we can give a drug that specifically attacks that gene (or the later consequences of that gene and thereby give a treatment that is more likely to work and less likely to have severe side effects.
Scientists at Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) have been leaders in the research to systematically test large numbers of mutations and identify the genetic culprits in cancer cells. The genotyping has led to many new discoveries regarding the cause of cancer and has paved the way to design new clinical trials. A large number of these clinical trials take advantage of new drugs designed to attack specific genetic changes.
“The right dose of the right drug, for the right patient, at the right time” is the mantra of precision medicine.
Genetic Mutations and Cancer
The development and spread of every cancer is driven by a unique set of abnormalities in its genetic makeup – these are present only in the tumor and not in normal tissues. The complete set of genetic information, written in DNA code, is present in each cell’s genes and chromosomes. So while different patients may be diagnosed with what appears to be the same type of cancer (e.g., lung cancer), the exact genetic makeup of the tumor varies from patient to patient.
“We believe the way a cancer behaves is driven largely by changes in its DNA (genes or chromosomes), as well as other abnormalities,” says Neal I. Lindeman, MD, director of the Center for Advanced Molecular Diagnostics at Brigham and Women’s Hospital. “Precision cancer medicine aims to fully characterize the changes present in the cancers, so that treatment is targeted and effective against the molecular changes in each of the different cancers, and at the same time less toxic to normal cells in the body.”
Precision cancer medicine utilizes molecular diagnostic testing, including DNA sequencing, to identify cancer-driving abnormalities in the tumor’s genome. By defining the impact of the diverse genetic abnormalities, scientists can begin to identify treatments directed against these genetic abnormalities and develop new approaches to combat the cancers. Through comprehensive genomic characterization, clinical trials directed against these genetic abnormalities, molecular imaging to assess the response to treatment, and other tailored care approaches, precision cancer medicine can provide effective treatment options that are based on an individual patient’s unique DNA profile.
“Once we identify and analyze the cancer-causing abnormalities, the clinically relevant information can be shared with your cancer team. The information about the specific cancer-causing changes can then be used to identify the best drug or combination of drugs to use,” says Dr. Lindeman.
Targeted therapies are designed to attack specific mutations and other cancer-related changes in the DNA programming of cells. Mutations are changes in the DNA or genetic code that serves as the cell’s operating manual; many mutations can be found in cancer cells. Other types of cancer-causing changes are pieces of DNA that are deleted, excessively copied (also referred to as amplification), the loss of large segments of DNA, and events that cause two pieces of DNA to swap positions on the chromosomes and therefore alter the activity, function amount or spatial location of a gene product.
Types of Mutations
Most cancers are caused and maintained by DNA mutations that aren’t present in normal tissues but can occur in organs at risk for cancer during one’s lifetime. These acquired or somatic mutations aren’t present in normal DNA so are not inherited or passed on to offspring. Many somatic mutations cause molecular cell-growth pathways to be activated, rather than carefully regulated as the pathways are turned on and off as needed in normal cells. The activation of the molecular cell-growth pathways lead to endless proliferation and cancer spread. Genes affected by these kinds of mutations are called oncogenes. Although we have two copies of nearly all genes, only one copy of an oncogene needs to be mutated to cause a cancer.
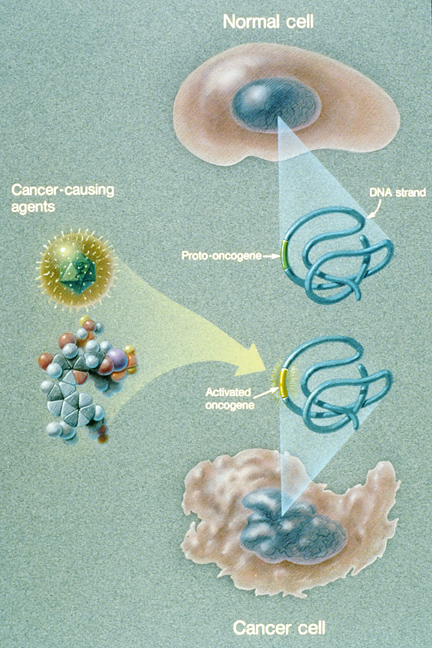
This illustration explains how a normal cell becomes a cancer cell. An oncogene in a normal cell appears to regulate and influence cell growth and division. When a cancer causing agent affects a cell’s DNA and the oncogene is activated, a cancer cell develops. (Courtesy of the National Cancer Institute)
Other mutations disable genes that normally suppress growth or the development of a tumor. These so-called tumor-suppressor genes normally act as brakes on excessive cell growth. Once these tumor-suppressor genes are inactivated or disabled, the cells are released from the growth suppression and grow uncontrollably. Most cancers are driven by several mutations that can include both growth-stimulating cancer causing oncogenes as well as damaged tumor-suppressor genes that release the cells from growth control.
Cancers can also run in families, these are known as familial cancer syndromes. The cancers that occur more frequently in close family members are caused by germline mutations that occur in the DNA of parental sperm or eggs and are passed on to offspring, who then carry the mutation in every cell. Most of these are tumor suppressor genes in which every cell in the body has one dysfunctional copy (either due to a mutation or a deletion); whenever the one normal copy gets altered by a new mutation later in life (either due to an environmental exposure or a random mutation) then a cancer can grow. For this reason, patients with these syndromes often have cancers in more than one organ at multiple times in their lives.
For example, the BRCA1 and BRCA2 breast and ovarian cancer susceptibility gene mutations are carried within families. Mutations in another gene called TP53 cause Li-Fraumeni familial cancer syndrome. Individuals with this syndrome are at risk for developing breast, soft tissue, brain, bone, blood, and other cancers. These are examples of germline mutations that can predispose close relatives to different cancers if the particular gene mutation is inherited.
If doctors suspect that an unusual number or pattern of cancers within a family may be caused by an inherited cancer predisposition gene that is passed from family members, specialists from Dana-Farber’s Center for Cancer Genetics and Prevention can provide counseling and offer testing to find out if an individual carries an inherited cancer gene.
Dana-Farber/Brigham and Women’s Cancer Center Contributions
Scientists at Dana-Farber Cancer Institute and Brigham and Women’s Hospital have extensive experience in precision cancer medicine. Four decades ago, discoveries made around the world helped provide a better understanding of the genetic and biological underpinnings of breast cancer. Breast cancer can be driven by the hormones estrogen and progesterone, or by an overabundance of a protein called HER-2 that is caused by the breast cancer acquiring multiple copies of the HER2 gene. This laid the foundation for the development of targeted treatments directed against the estrogen and progesterone receptors and HER2 that have worked successfully for millions of women with breast cancer.
Edward Benz, MD, President and Chief Executive at Dana-Farber Cancer Institute, presents a TEDx talk about precision cancer medicine
A decade ago, Dana-Farber researchers helped launch the era of precision cancer medicine for lung cancer through a landmark study that discovered a dramatic response to gefitinib (Iressa), a new treatment targeting a malfunctioning protein called the epidermal growth factor receptor or EGFR. Investigators at DF/BWCC further showed that the malfunctioning protein is caused by specific mutations within the area that bind the drug, making it particularly effective in the subset of patients with lung cancer and an EGFR mutation. DF/BWCC further extended this work by offering the world’s first clinical lab test for EFGR mutations, expanding access to these treatments for patients in routine clinical care and not just in research trials.
Earlier this year, in another study of patients with lung cancer, more information about the power of personalized therapies was unleashed. DF/BWCC investigators led a study performed in 14 different institutions that found treatments matched to specific genomic abnormalities in advanced lung cancer patients yield a year longer survival, on average, compared to the lung cancer patients treated with standard therapies. Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and other collaborators this year also discovered potential drug targets for different childhood brain tumors, such as juvenile astrocytomas and craniopharyngiomas.
An extensive DF/BWCC research project called Profile is building a comprehensive knowledge base of cancer-related mutations and other DNA alterations detected in tumor samples of participating patients. If the genetic test finds mutations that are “clinically actionable” today (that is, there are potential targets for drug therapies or information helpful in predicting the cancer’s course), the results are provided to the patient’s physician for further action, which could involve offering the patient enrollment in a clinical trial or a prescription for an already identified and approved drug treatment.
“There’s been a major evolution in cancer treatment,” says Bruce Johnson, MD, chief clinical research officer at Dana-Farber Cancer Institute. “We no longer think only about where a cancer arises: that is, in the prostate, in the blood cells, in the kidneys. Now we think about the unique genetic changes that drive your cancer – and how precisely we can build the tools to stop them.”



