“The purpose of precision cancer medicine is not to categorize or classify cancers solely by site of origin, but to define the genomic alterations that are driving that cancer,” says Laura MacConaill, PhD, scientific director of the Profile Research Project and scientific director of the Center for Cancer Genome Discovery. “Even if an alteration occurs in a given cancer type only 1 percent of the time, and you can provide an effective treatment option for those specific patients, it’s incredibly enabling and powerful.”
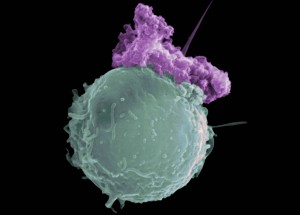
Apoptosis is the process of programmed cell death (PCD) that may occur in multicellular organisms.
What’s Normal
Normal cells have control systems that allow them to replicate only as needed for growth – in children, for example – or to replace damaged or worn-out cells. Most normal cells are firmly anchored to one area of the body, such as the lung or the breast. When damage to a cell cannot be repaired or when a cell reaches the end of its useful life span, it is programmed to die, a process called apoptosis.
What’s Not Normal
Cancer cells don’t follow these rules. Gene mutations, chromosome alterations, and changes in other molecules enable them to replicate endlessly, ignoring signals ordering them to self-destruct, and to migrate to distant sites in the body, such as the bones, liver, or brain, where they set up satellite tumors. Usually, several genomic abnormalities are driving the cancer, but these can be different in different cancers that otherwise seem to be the same. Two people with the same type of lung cancer, for example, may not respond to treatment in the same way if their cancers are driven by different combinations of mutations.
Cancer-driving abnormalities may occur spontaneously (de novo) or be passed from parent to child (hereditary). Spontaneous changes happen all the time – thousands of times a day in single cell – often due to DNA damage caused by factors like sunlight, diet, or smoking. Some abnormalities are harmless, some cause the cell with the mutation to die, and some are fixed by the body’s elaborate repair mechanisms or are eliminated before cell replication occurs. A very small minority of these changes, however, can evade repair and alter the cell in a way that, rather than die, develops an improved ability to grow or survive. These alterations lead to cancer.

Three classes of gene mutations
Three classes of gene alterations can drive cancer. The first, referred to as a SNV (single nucleotide variation) or “point mutation” is a qualitative change that affects a single base nucleotide – a “letter” of DNA code (A, G, T or C) – and changes it to a different letter base (i.e., a T to A change). It may cause the gene to direct the cell to make an abnormal protein (a “missense” mutation) or may prevent the gene from making a protein at all (a “nonsense” mutation). Although human cells have multiple safeguards to protect them against gene mutations, it is likely that several genes are defective if an invasive cancer develops.
The second class of gene alteration is copy number variation, which is a quantitative alteration. Each of us typically has two identical copies of each gene. A copy number alteration (CNV) is when one or both of those copies are lost (“deletion”) or when extra copies are acquired (“amplification”). Losses and gains can co-exist with SNVs; for example, a tumor might lose one copy of a tumor suppressor gene and have a mutation in the second copy. Gains and losses can be within a gene, involve a whole gene, large clusters of genes, whole chromosome regions, entire chromosomes or even the entire genomic content of the cell (“haploidy” and “polyploidy”). As with SNVs, CNVs can either activate an oncogene or silence a tumor suppressor gene.
The third class of alteration is a structural alteration (SV). In this class, a chromosome may have broken and segments become rearranged or swapped with pieces of other chromosomes (“translocation”) or the same chromosome (“inversion”). An extreme version of this, chromothripsis, involves shattering the chromosomes into many fragments that then reassemble with many broken genes. When the chromosome breaks and rejoins (“breakpoints”), those genes are altered. In this setting, completely new functions can arise or gene products can be turned on in places or at times where they should not.
Treating Abnormalities That Are Cancerous
Standard chemotherapy typically destroys rapidly dividing cells, normal and cancerous in a wide range of tissues, often causing side effects like nausea, mouth sores, and hair loss. By contrast, precision cancer medicine uses targeted therapies engineered to attack tumor cells with specific abnormalities. Such medications block the growth and spread of cancer while leaving normal cells largely unharmed. Some agents are designed to strike directly at cells with specific genetic changes that drive tumors’ development and survival, or to inhibit overactive signaling pathways that allow cancer cells to grow and divide uncontrollably. Other treatments enlist the immune system to identify and fight the cancer cells.



