One hallmark of Dana-Farber/Brigham and Women’s Cancer Center is the close integration between the clinical care of our patients and our research labs, with each informing the other. The introduction of genomics has accelerated this process and has already led to dramatic advances in our understanding of the causes and makeup of cancer, and most importantly, to exciting new “personalized” therapies for the benefit of our patients.
The mapping of the human genome advanced our understanding of the genetic alterations associated with different cancers. Scientists can now interrogate each individual’s specific cancer, and consequently, have uncovered many of cancer’s underlying causes and behavior.

“We practice team science. We work with industry, regulatory authorities, and funding agencies. But most important, our patients are the heroes, inspiration, and reason for all we do.” – Kenneth Anderson, MD, director of the Jerome Lipper Multiple Myeloma Center
While these basic mechanisms are widely shared among cancers, we have learned that each patient’s tumor is unique and can have different combinations of mutations, even for the seemingly same type of cancer. It is clear that successful cancer treatment will require more than a simple attack on the cancer cells themselves with a single drug but will likely require multiple different types of therapy. Ultimately, our objective is to provide the most precise cancer care for patients, delivering the most tailored and effective treatments based on each patient’s unique tumor makeup.
We now know that there are many more forms and subtypes of cancer than originally believed, with more commonalities among cancers previously thought to be unrelated. This new ability to classify cancers by the genetic mutations that cause them, rather than by the part of the body in which they appear, helps us to develop better and more precise therapies.
Dana-Farber Cancer Institute and Brigham and Women’s Hospital conduct a wide range of cancer research and continue to invest in the latest cancer research disciplines and technologies. They are also part of the Dana-Farber/Harvard Cancer Center (DF/HCC), which is the largest comprehensive cancer center in the world. Our combined expertise and resources have been critical to advancing the field of precision cancer medicine.
Understanding Cancer
Researchers across Dana-Farber Cancer Institute and Brigham and Women’s Hospital knit multiple perspectives together into a more detailed understanding of the basic biology of cancer.
The breadth and depth of our cancer research efforts are extensive, and can range from more basic cellular and biologic research about the human body to more focused molecular and genomic research that identifies the genetic makeup of cancer. All of these various types of research are critical to understanding more about the precise causes and makeup of cancer, and have already enabled us to find and continue to find cancer biomarkers, which inform clinicians about the presence of cancer, help determine a prognosis, and predict how well a patient will respond to treatment.
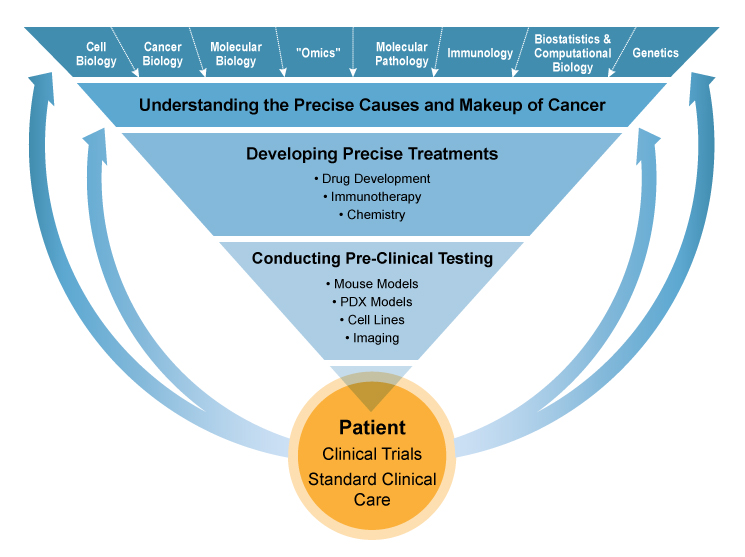 The path from understanding what causes cancer to using that information to guide clinical care is complex and iterative. Precision cancer medicine research is cumulative, with each step informing the next in a cycle of discovery and development. Scientists learn about previously unknown causes and makeup of cancer, which helps them to identify potential genetic targets for new therapies. Chemical biologists and pharmaceutical companies develop compounds capable of blocking those targets. Clinical investigators and clinicians administer and test new agents in patients on clinical trials. The results of these trials then inform all of the previous stages to better define drug targets and understand cancer response to drugs, and the cycle begins anew.
The path from understanding what causes cancer to using that information to guide clinical care is complex and iterative. Precision cancer medicine research is cumulative, with each step informing the next in a cycle of discovery and development. Scientists learn about previously unknown causes and makeup of cancer, which helps them to identify potential genetic targets for new therapies. Chemical biologists and pharmaceutical companies develop compounds capable of blocking those targets. Clinical investigators and clinicians administer and test new agents in patients on clinical trials. The results of these trials then inform all of the previous stages to better define drug targets and understand cancer response to drugs, and the cycle begins anew.
Key to this work is Profile one of the nation’s most comprehensive personalized cancer medicine initiatives. With Profile, scientists are creating one of the world’s largest databases on the genetic abnormalities that are associated with the many types of tumors, as well as advancing the goals of personalized, precision cancer care.
More than 31,000 patients have consented to have their tumor tissue analyzed for the presence of mutations and other cancer-related DNA abnormalities. Since its inception, Profile has completed more than 10,000 genetic profiles of patients’ tumors, adding about 400 each month to the database.
Genomics and molecular pathology research has been particularly important in advancing precision cancer research over the past few years. By cataloging the genomic composition and changes of cancer cells, we are now able to identify and better predict cancer behavior.
In partnership with the Broad Institute of MIT and Harvard, Dana-Farber’s Center for Cancer Genome Discovery developed innovative DNA sequencing technology to reveal genome alterations and abnormalities in multiple types of cancer types.
Developing Precise Treatments and Conducting Pre-Clinical Testing
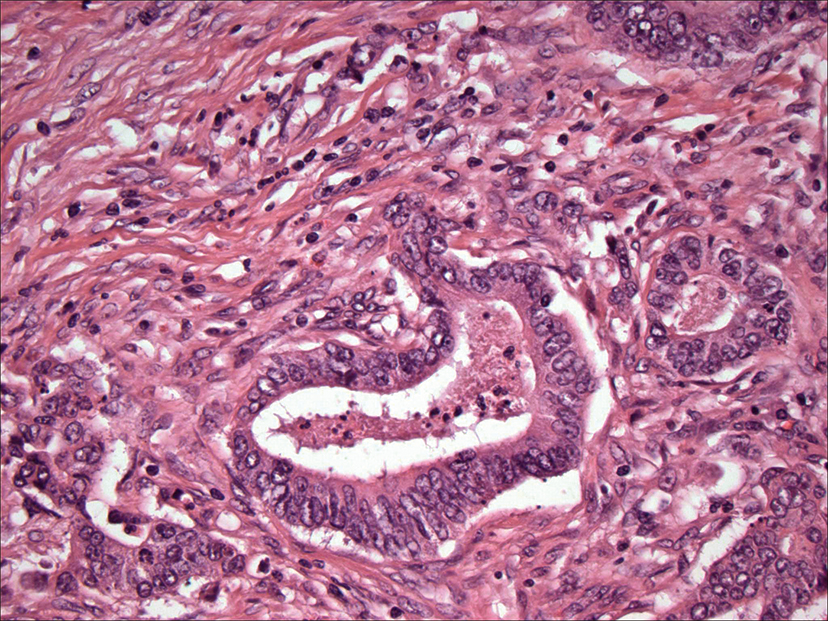
An image of colon cancer with cancer cells forming circled structures
As researchers get a fuller picture of the biomarkers and genetic abnormalities associated with different types of cancer, they are able to identify which of these abnormalities make the best targets for treatment and develop more tailored and precise treatments for specific mutation types. These may come in the form of newly developed drugs, immunotherapies, and other approaches – or a combination of multiple therapies. For example, some drugs may be more effective in combination with selected immunotherapies.
Based on the type of biomarkers that exist in different types of tumors, researchers can then develop drugs that pinpoint specific targets (i.e., the cancer mutations) to stop, delay or decrease the production of cancer cells. Biomarkers can sometimes serve as “surrogate endpoints” to monitor a drug’s effect on cancer progression and survival.
Once a treatment has been developed, researchers then test it in various tumor models (animal, PDX, cell lines, etc.) before administering the drug to a patient. Drugs are monitored through advanced imaging and other laboratory techniques to understand how tumors (and their mutations) react to different treatments before introducing it as a clinical trial.
Clinical Trials
Clinical trials are scientific studies in which new treatments – drugs, diagnostic procedures, and other therapies – are tested in patients to determine if they are safe and effective. DF/BWCC offers a broad set of clinical trials for many cancer types.
On some clinical trials, if a patient consents, investigators can link genetic information about each tumor with data from the individual’s medical records to track how the disease responds to treatment, the type and severity of side effects, and other health-related issues. This information can help determine which therapies are most effective against certain cancers, and which doses and treatment schedules produce the best results.
Regardless of the clinical trial type, findings from each clinical trial are fed back into the research pipeline to better understand cancer behavior and refine therapeutic targets. Comprehensive genomic data, combined with clinical trial data showing how patients respond to different cancer treatments, helps identify patterns that can improve targeted treatments. This process, known as systematic genotyping, accelerates the development of new therapies and is at the heart of precision cancer medicine.



